THE CONTEXT: The Nobel Prize in Chemistry for 2020 has been awarded for the discovery of CRISPR Cas9. The two scientists have pioneered the use of CRISPR – Cas9 (CRISPR-associated protein 9) system as a gene-editing tool. This article discusses about the gene editing, CRISPR-Cas9 technique and concern related to this.
UNDERSTANDING GENE EDITING
Genes contain the bio-information that defines any individual. Physical attributes like height, skin or hair colour, more subtle features and even behavioural traits can be attributed to information encoded in the genetic material. An ability to alter this information gives scientists the power to control some of these features.
- Gene editing is also called as genetic modification, genetic manipulation or genetic engineering.
- Genome editing is a group of technologies that give scientists the ability to change an organism’s DNA (Deoxyribonucleic acid).These technologies allow genetic material to be added, removed, or altered at particular locations in the genome.
- Gene Editing is widely practised in agriculture, to increase productivity or resistance to diseases, etc.
WHAT IS CRISPR?
- Gene editing technology-Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) is a gene editing technology, which replicates natural defence mechanism in bacteria to fight virus attacks, using a special protein called Cas9.
- CRISPRs are specialized stretches of DNA. The protein Cas9(or “CRISPR-associated”) is an enzyme that acts like a pair of molecular scissors, capable of cutting strands of DNA. It allows researchers to easily alter DNA sequences and modify gene function.
- Cut-and-paste mechanism-CRISPR-Cas9 technology behaves like a cut-and-paste mechanism on DNA strands that contain genetic information. The specific location of the genetic codes that need to be changed, or edited, is identified on the DNA strand, and then, using the Cas9 protein, that location is cut off from the strand.
WHY IS CRISPR- CAS9 SYSTEM SIGNIFICANT?
- Normally, if sperm from a father with one mutant copy of the gene is fertilized in vitro with normal eggs, 50% of the embryos would inherit the condition.However, when the gene-editing tool was used, the probability of inheriting the healthy gene increased from 50 to 72.4%.
- Clinical trials are under way in many countries to use this tool for treating cancer, cystic fibrosis, haemophilia, and sickle cell disease.
- It was shown in mice that it is possible to shut down HIV-1 replication and even eliminate the virus from infected cells.
- In agriculture, a new breed of crops that are gene-edited will become commercially available in a few years.
- It is the simplest yet powerful tool for editing genomes and also termed as the most versatile and precise method of genetic manipulation.
GUIDELINES AND REGULATIONS IN INDIA
- In India, several rules, guidelines, and policies are notified under the Environment Protection Act, 1986 to regulate genetically modified organisms.
- The above Act and the National Ethical Guidelines for Biomedical and Health Research involving human participants, 2017, by the Indian Council of Medical Research (ICMR), and the Biomedical and Health Research Regulation Bill implies regulation of the gene-editing process.
This is especially so in the usage of its language “modification, deletion or removal of parts of heritable material”.However, there is no explicit mention of the term gene editing.
ETHICAL CONCERN
- Last year, a Chinese researcher used the tool to modify a particular gene in the embryo to make babies immune to HIV infection, which led to international furore. Though no guidelines have been drawn up so far, there is a general consensus in the scientific and ethics communities that the gene-editing technique should not be used clinically on embryos.
- It would be a profound leap of science and ethics. This kind of gene editing is banned in most countries as the technology is still experimental and DNA changes can pass to future generations, potentially with unforeseen side-effects.
- Another ethical challenge germline cell and embryo genome editing (Making genetic modifications to human embryos and reproductive cells such as sperm and eggs is known as germline editing) brings up includes whether it would be permissible to use this technology to enhance normal human traits (such as height or intelligence).
- There are growing concerns of trying to produce “designer” babies or altered human beings.
OTHER CONCERNS
- Variable efficacy– CRISPR technology is not hundred percent efficient. The genome-editing efficiencies can vary.
- Off-target effects and imprecise edits- There is also the phenomenon of “off-target effects,” where DNA is cut at sites other than the intended target. This can lead to the introduction of unintended mutations. Furthermore, even when the system cuts on target, there is a chance of not getting a precise edit. This is called as “genome vandalism.”
- There is also a potential ecological impactof using gene drives. An introduced trait could spread beyond the target population to other organisms through crossbreeding. Gene drives could also reduce the genetic diversity of the target population.
WAY FORWARD
- The World Health Organization formed a panel of gene-editing experts.The expert panel suggested a central registry of all human genome editing research in order to create an open and transparent database of ongoing work.
- Experts recommend that germline editing should be done only on genes that lead to serious diseases and when no other reasonable treatment alternatives exist.
- Among other criteria, they stress the need to have data on the health risks and benefits and the need for continuous oversight during clinical trials. They also recommend following up on families for multiple generations.
- CRISPR technology is indeed a path-breaking technology, to alter genes in order to tackle a number of conventional and unconventional problems. The most promising use of the CRISPR technology is in treatment of diseases. For example, sickle cell anaemia.
- However, experiments and tests to validate its use must be subjected to appropriate scrutiny by the regulators, and their use must be controlled to prevent commercial misuse.
- Scientists across the world are still working to determine whether the CRISPR technology is safe and effective for use in people.
CONCLUSION:
The huge potential to edit genes using this tool has been used to create a large number of crop varieties with improved agronomic performance; it has also brought in sweeping changes to breeding technologies. The gene-editing tool has indeed taken “life sciences into a new epoch”. Therefore it is time that India should come up with a specific law to monitor germline editing and guidelines for conducting gene-editing research, which will give rise to modified organisms while sorting ethical concerns.
JUST TO ADD IN YOUR KNOWLEDGE
UNDERSTANDING THE MECHANISM
The CRISPR-Cas9 system consists of two key molecules that introduce a change (mutation) into the DNA. These are:
- An enzyme called Cas9. This acts as a pair of ‘molecular scissors’ that can cut the two strands of DNA at a specific location in the genome so that bits of DNA can then be added or removed.
- A piece of RNA called guide RNA (gRNA). This consists of a small piece of pre-designed RNA sequence
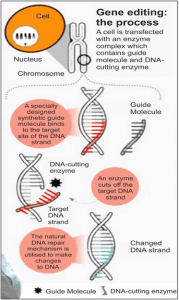 (about 20 bases long) located within a longer RNA scaffold. The scaffold part binds to DNA and the pre-designed sequence ‘guides’ Cas9 to the right part of the genome. This makes sure that the Cas9 enzyme cuts at the right point in the genome.
(about 20 bases long) located within a longer RNA scaffold. The scaffold part binds to DNA and the pre-designed sequence ‘guides’ Cas9 to the right part of the genome. This makes sure that the Cas9 enzyme cuts at the right point in the genome. - The guide RNA is designed to find and bind to a specific sequence in the DNA. The guide RNA has RNA bases that are complementary to those of the target DNA sequence in the genome. This means that the guide RNA will only bind to the target sequence and no other regions of the genome.
- The Cas9 follows the guide RNA to the same location in the DNA sequence and makes a cut across both strands of the DNA.
- At this stage the cell recognises that the DNA is damaged and tries to repair it.
- Scientists can use the DNA repair machinery to introduce changes to one or more genes in the genome of a cell of interest.

