THE CONTEXT: Ever since the first vaccine was developed in 1796 to treat smallpox, several different methods have been created to develop successful vaccines. Today, those methods, known as vaccine technologies, are more advanced and use the latest technology to help protect the world from preventable diseases. Depending on the pathogen (a bacteria or virus) that is being targeted, different vaccine technologies are used to generate an effective vaccine. Just like there are multiple ways to develop a vaccine, they can also take on multiple forms—from needle injections and nasal sprays to oral doses, a more recent innovation. In total, there are six different vaccine technology platforms, each with its own benefits, and examples.
VACCINE
A vaccine is a biological preparation that improves immunity to a particular disease. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins or one of its surface proteins. Vaccines are like a training course for the immune system. They prepare the body to fight disease without exposing it to disease symptoms.
How a vaccine works – a general overview:
- When viruses or bacteria (germs) invade our body, they attack and multiply. This invasion is called an infection, and the infection is what causes illness.
- The first time the body encounters a germ, it can take several days for the immune system to make and use all the tools it needs to fight the infection.
- After the infection has been eradicated, the immune system keeps a few “memory cells” that remember what it learned about how to protect against that disease.
- If the body encounters the same virus or bacteria again, it will produce antibodies to attack the germ more quickly and efficiently.
TYPES OF VACCINES
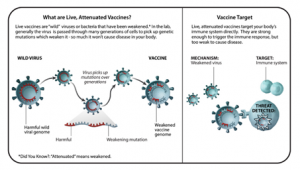
LIVE-ATTENUATED VACCINES
- Live-attenuated vaccines contain live pathogens from either bacteria or a virus that have been “attenuated,” or weakened. Live-attenuated vaccines are produced by selecting strains of a bacteria or virus that still produce a robust enough immune response but that does not cause disease.
- Attenuated viruses were one of the earliest methods of eliciting protective immune responses.
- “Vaccinia, the first-ever vaccine which protects against smallpox, is actually where we get the term ‘vaccination’ from.”
- Benefits: Because these types of vaccines contain a live pathogen, the immune system reacts very well to them and it will typically remember the pathogen for a very long time. Additional doses, or booster shots, are not always needed.
- Examples: Measles, mumps, and rubella (MMR) vaccine, varicella (chickenpox) vaccine.
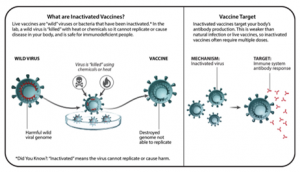
INACTIVATED VACCINES
- Inactivated vaccines take a live pathogen and inactivate or kill it. When the vaccine is then introduced to a human through a shot, for example, the inactivated pathogen is strong enough to create an immune response, however, is incapable of causing disease. Multiple doses are often needed in order to build up immunity and offer full protection.
- Benefits: Inactivated vaccines can be mass-produced and are relatively inexpensive to make.
- Examples: Polio vaccine, influenza vaccine
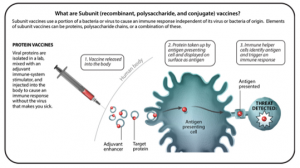
SUBUNIT VACCINES
- Subunit vaccines are made from a piece of a pathogen, not the whole organism, so they do not contain any live pathogens. Some important subunit vaccines are polysaccharide vaccines, conjugate vaccines, and protein-based vaccines.
- Polysaccharide vaccines target an immune response to pathogenic bacteria that are encased in a layer of sugar. This means they help you make protective responses against the surface of the bacteria, allowing your body to kill the bacteria. These do not work and therefore are not used for children under the age of 2 years.
- Conjugate vaccines are the same in that they have a polysaccharide component, but that sugar is stuck to a protein so your immune system will respond to the sugar on the bacteria better. They also help your body remember the bacteria better, so if you get infected in the future, the immune response will be better. Importantly, these vaccines do work in children under 2 years of age.
- Protein-based vaccines allow you to make a protective response against a protein on the surface of a virus, against a protein on the surface of a bacteria, or against a secreted toxin. In this case, the immune response is against the protein components of the bacteria or virus, not the sugar coat. Certain proteins on the surface of bacteria or viruses help the pathogen cause disease, so inducing an immune response against them can help the body fight against the infection or the effects of the toxin.
- Subunit vaccines can be made one of two ways – from the original pathogen or recombinantly. Recombinant vaccines use another organism to make the vaccine antigen.
- Benefits: Subunit vaccines only contain pieces of a pathogen, not the whole organism, so they cannot make you sick or cause infection. This makes them suitable for people who should not receive “live” vaccines, such as young children, older people, and immunocompromised people.
- Examples: Haemophilus influenzae type B (Hib) vaccine (conjugate), pneumococcal vaccine (polysaccharide or conjugate), shingles vaccine (recombinant protein), hepatitis B (recombinant protein), acellular pertussis, MenACWY (conjugate).
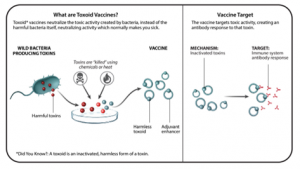
TOXOID VACCINES
- Toxoid vaccines use inactivated toxins to target the toxic activity created by the bacteria, rather than targeting the bacteria itself. The goal of toxoid vaccines is to give people a way to neutralize those toxins with antibodies through vaccination.
- Benefits: Toxoid vaccines are especially good at preventing certain toxin-mediated diseases such as tetanus, diphtheria, and pertussis. Booster shots are typically recommended every 10 years or so.
- Examples: Tetanus vaccine, diphtheria vaccine
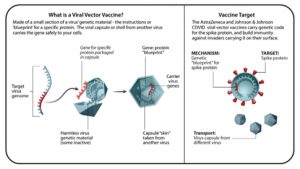
VIRAL VECTOR VACCINES
- Viral vector vaccines use a harmless virus to deliver to the host cells the genetic code of the antigen you want the immune system to fight. They are basically a gene delivery system. In doing so, information about the antigen is delivered, which triggers the body’s immune response.
- Benefits: Viral vector vaccines usually trigger a strong immune response. Typically, only one dose of the shot is needed to develop immunity. Boosters may be needed to maintain immunity.
- Examples: Ebola vaccine, COVID-19 vaccine (AstraZeneca and Johnson & Johnson)
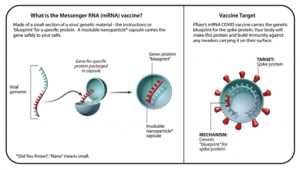
MESSENGER RNA (MRNA) VACCINES
- One of the newest and most exciting areas in vaccine technology is the use of mRNA vaccines. Unlike conventional vaccines—which can take many months or even years to cultivate—mRNA vaccines can be developed quickly using the pathogen’s genetic code. mRNA is likely to be at least one of the waves of the future for vaccines.
- When an mRNA vaccine is delivered, the RNA material teaches our body how to make a specific type of protein that is unique to the virus but does not make the person sick. The protein triggers an immune response, which includes the generation of antibodies that recognize the protein. That way, if a person is ever exposed to that virus in the future, the body would have the tools (antibodies) to fight against it.
- Benefits: It is a very powerful technique to be able to create a lot of a vaccine fast. The benefit is that the technology is very adaptable. We can potentially go in and change the mRNA in the formulation to target a new antigen and can make a lot of high-quality vaccine material relatively quickly.
- Examples: Pfizer-BioNTech COVID-19 vaccine
mRNA VACCINE AND CONVENTIONAL VACCINE
CONVENTIONAL VACCINE
PRODUCTION TIME
- Most vaccines against viral diseases are made from viruses grown in chicken eggs or mammalian cells.
- The process of collecting the viruses, adapting them to grow in the lab, and shipping them around the world can take months and is complex.
- For newly emerging viruses like SARS-CoV-2, for which a new vaccine is needed as quickly as possible, these steps may slow down development.
BIOSAFETY
- Growing large quantities of viruses to make each batch of vaccines creates potential hazards.
IMMUNE RESPONSE
- The antigen (a piece of the virus) is injected into the body. Upon recognizing the antigen, the immune system produces specific antibodies in preparation for the next time the body encounters the pathogen.
FLEXIBILITY
- Each new vaccine requires a bespoke production process, including complex purification and testing.
Transport and storage temperature
- Various vaccines have different temperature requirements, and generally can be stored in common refrigerators.
mRNA VACCINE
PRODUCTION TIME
- The RNA (which encodes an antigen of the infectious agent) is made from a DNA template in the lab.
- The DNA can be synthesized from an electronic sequence that can be sent across the world in an instant by computer. Currently, it takes about a week to generate an experimental batch of an RNA vaccine.
BIOSAFETY
- No virus is needed to make a batch of an RNA vaccine.
- Only small quantities of viruses are used for gene sequencing and vaccine testing.
IMMUNE RESPONSE
- The RNA is injected into the body and enters cells, where it provides instructions to produce antigens. The cell then presents the antigens to the immune system, prompting T-cell and antibody responses that can fight the disease.
FLEXIBILITY
- It is anticipated that the production process for RNA vaccines may be scaled and standardized; potentially enabling the replacement of the sequence encoding the target protein of interest for a new vaccine, with minimal changes to the vaccine production process.
Transport and storage temperature
- Requires sub-zero temperature which makes them hard to transport and make available at all places. Generally -20 to -70 degree celsius.
ADMINISTRATION OF VACCINES
ORAL VACCINE (PO)
The oral vaccine is administered through drops to the mouth. Rotavirus vaccine (RV1 [Rotarix], RV5 [RotaTeq]) is the only routinely recommended vaccine administered orally only. Rotavirus vaccine should never be injected. Though Polio vaccine is given in two ways: an inactivated poliovirus given by injection and a weakened poliovirus given by mouth.
INTRANASAL ROUTE (NAS)
The intranasal vaccine is administered into each nostril using a manufacturer-filled nasal sprayer. Live, attenuated influenza (LAIV [FluMist]) vaccine is administered by the intranasal route.
SUBCUTANEOUS ROUTE (SUB CUT)
Subcutaneous injections are administered into the fatty tissue found below the dermis and above muscle tissue.
INTRAMUSCULAR ROUTE (IM)
Intramuscular injections are administered into the muscle through the skin and subcutaneous tissue. The recommended site is based on age. Use the correct needle length and gauge based on the age, weight, and gender of the recipient.
Previous Year Questions:
Q1.UPSC CES PRE – 2022
In the context of vaccines manufactured to prevent the COVID-19 pandemic, consider the following statements:
- The Serum Institute of India produced a COVID-19 vaccine named Covishield using an mRNA platform.
- Sputnik V vaccine is manufactured using vector-based platform.
- COVAXIN is an inactivated pathogen-based vaccine.
Which of the statements given above are correct?
a) 1 and 2 only
b) 2 and 3 only
c) 1 and 3 only
d) 1, 2 and 3
Q2.UPSC CDS 1 – APRIL 2022
Consider the following pair of vaccines and category/type:
- Covaxin – Inactivated pathogen based vaccine.
- Covishield – mRNA vaccine.
- Sputnik V – Viral vector based vaccine.
Which of the pairs given above is/are correct?
a) 1 only
b) 2 and 3 only
c) 1 and 3 only
d) 1, 2 and 3
Mains Practice Question:
- Explain the various technologies used for making vaccines.
- Write a note mRNA vaccine and how it scores over the conventional vaccines.
COVID VACCINES APPROVED FOR USE IN INDIA
VACCINE COMPANY TYPE ADMINISTRATION TYPE 1. Corbevax Biological E Ltd Protein Subunit INTRAMUSCULAR ROUTE (IM) 2. COVOVAX Serum Institute of India Protein Subunit INTRAMUSCULAR ROUTE (IM) 3. ZyCov-D Zydus Cadila DNA WORLD’S FIRST NEEDLE-FREE PLASMID DNA VACCINE. 4. GEMCOVAC Gennova Biopharmaceuticals Limited RNA INTRAMUSCULAR ROUTE (IM) 5. Spikevax Moderna RNA INTRAMUSCULAR ROUTE (IM) 6. Sputnik Light Gamaleya Non Replicating Viral Vector INTRAMUSCULAR ROUTE (IM) 7. Sputnik V Gamaleya Non Replicating Viral Vector INTRAMUSCULAR ROUTE (IM) 8. Ad26.COV2.S Johnson & Johnson Non Replicating Viral Vector INTRAMUSCULAR ROUTE (IM) 9. Vaxzevria Oxford/AstraZeneca Non Replicating Viral Vector INTRAMUSCULAR ROUTE (IM) 10. Covishield (Oxford/ AstraZeneca formulation) Serum Institute of India Non Replicating Viral Vector INTRAMUSCULAR ROUTE (IM) 11. Covaxin Bharat Biotech Inactivated INTRAMUSCULAR ROUTE (IM)




