Next-Generation Sequencing (NGS) is a revolutionary technology that allows for the rapid sequencing of DNA and RNA, enabling researchers to analyze genetic material at an unprecedented scale and speed. Unlike traditional sequencing methods, such as Sanger sequencing, which can only sequence a single DNA fragment at a time, NGS can sequence millions of fragments simultaneously, providing vast amounts of data in a short period. This capability has transformed genomics, personalized medicine, and various fields of biological research.
Features of NGS
1. Massive Parallel Processing: NGS utilizes massively parallel sequencing technologies that allow multiple sequences to be analyzed simultaneously. This results in significantly reduced time and cost compared to traditional methods.
2. High Throughput: NGS platforms can generate gigabases to terabases of data in a single run, enabling comprehensive genomic studies that were previously impractical.
3. Cost-Effectiveness: The cost of sequencing has dramatically decreased over the years. For example, the cost to sequence an entire human genome has dropped from approximately $100 million in 2001 to around $1,000 today.
4. Versatility: NGS can be applied to various types of sequencing, including whole-genome sequencing (WGS), targeted sequencing, exome sequencing (WES), and RNA sequencing (RNA-Seq), making it suitable for a wide range of applications.
How NGS Works?
The NGS process typically involves several key steps:
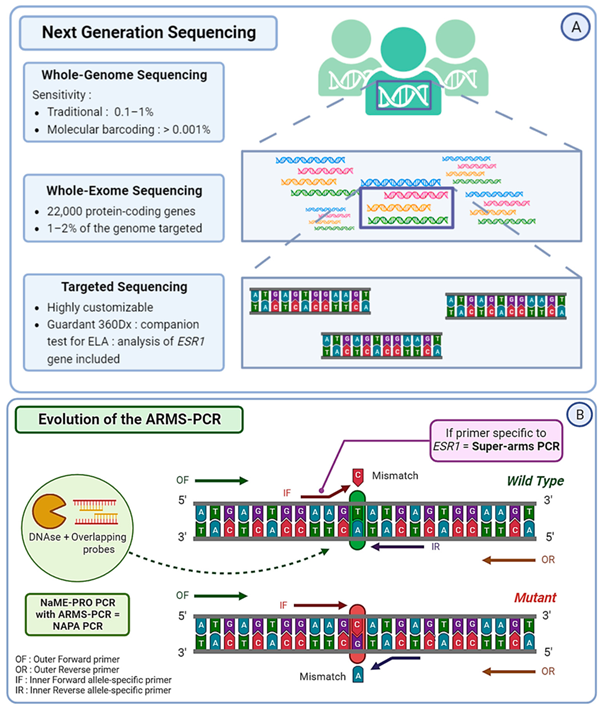
1. Sample Preparation: DNA or RNA is extracted from the sample and fragmented into smaller pieces. Adapters are ligated to the ends of these fragments to facilitate sequencing.
2. Library Construction: The prepared fragments are amplified to create a library of sequences that will be analyzed during the sequencing process.
3. Sequencing: The library is loaded onto a sequencing platform where millions of fragments are sequenced simultaneously using various techniques such as sequencing by synthesis (Illumina), pyrosequencing, or nanopore sequencing.
4. Data Analysis: The raw sequence data generated is processed using bioinformatics tools to align sequences, identify variants, and interpret results.
Applications of Next-Generation Sequencing
NGS has revolutionized many areas of research and clinical practice:
1. Genomic Research: NGS enables comprehensive studies of genomes, leading to discoveries related to genetic variations associated with diseases.
2. Personalized Medicine: By identifying specific genetic mutations in patients, NGS facilitates tailored treatment plans based on individual genetic profiles, especially in oncology.
3. Infectious Disease Surveillance: NGS is used for rapid identification and characterization of pathogens during outbreaks, aiding public health responses.
4. Cancer Genomics: It allows for the identification of mutations in tumor DNA, helping oncologists determine appropriate therapies based on the genetic makeup of tumors.
5. Microbiome Studies: NGS enables detailed analysis of microbial communities in various environments, including human gut microbiota, contributing to our understanding of health and disease.
6. Non-Invasive Prenatal Testing (NIPT): NGS technology is used in prenatal screening to detect genetic abnormalities in fetuses using maternal blood samples.
7. Pharmacogenomics: Understanding how genetic variations affect individual responses to drugs can lead to more effective and safer medication regimens.
Facts and Statistics about Next-Generation Sequencing
1. Cost Reduction Over Time: The cost per human genome sequenced has decreased from approximately $100 million in 2001 to about $1,000 as of 2021 .
2. Market Growth: The global next-generation sequencing market was valued at approximately $8 billion in 2020 and is projected to reach around $27 billion by 2026 , growing at a CAGR of about 23%.
3. Clinical Adoption Rates: A survey conducted by the National Human Genome Research Institute found that nearly 90% of clinical laboratories reported using some form of NGS technology by 2019 .
4. Applications in Oncology: According to a report by Grand View Research, the global cancer genomics market is expected to reach $19 billion by 2025 , with NGS playing a significant role in identifying actionable mutations for targeted therapies.
5. Impact on Infectious Disease Research: During the COVID-19 pandemic, NGS was instrumental in rapidly identifying SARS-CoV-2 variants; over 1 million genomes were sequenced globally by mid-2021 .
Challenges Facing Next-Generation Sequencing
Despite its advantages, several challenges remain:
1. Data Management and Analysis: The vast amounts of data generated by NGS require sophisticated bioinformatics tools for analysis and interpretation, which can be resource-intensive.
2. Interpretation of Variants: Determining the clinical significance of identified genetic variants remains challenging due to the complexity of genomic data and limited knowledge about many mutations.
3. Standardization and Quality Control: There is a need for standardized protocols and quality control measures across different laboratories to ensure consistent results.
4. Ethical Considerations: Issues related to privacy, consent, and data sharing must be addressed as genomic data becomes increasingly accessible.
Future Directions
The future of NGS is promising with ongoing advancements:
-
- Integration with Artificial Intelligence (AI): AI algorithms are being developed to enhance data analysis capabilities, improving accuracy in variant calling and interpretation.
- Long-Read Sequencing Technologies: Innovations such as PacBio and Oxford Nanopore Technologies are improving the ability to sequence longer DNA fragments, facilitating better assembly of complex genomes.
- Cost Reduction: Continued advancements in technology are expected to further reduce costs associated with sequencing.
- Expanded Clinical Applications: As understanding grows regarding the implications of genomic variations, NGS will likely find broader applications in clinical settings beyond oncology.
Conclusion
Next-Generation Sequencing represents a transformative leap in genomics that has opened new avenues for research and clinical applications. Its ability to generate vast amounts of data quickly and cost-effectively has made it an indispensable tool in modern biology and medicine. As technology continues to evolve, addressing challenges related to data management, interpretation, and ethical considerations will be crucial for maximizing the benefits of NGS in improving health outcomes worldwide.
Practice Questions for UPSC Mains Examination
Q.1 Discuss the impact of Next-Generation Sequencing (NGS) on personalized medicine and its implications for patient care in oncology.
Spread the Word



