TAG: GS-3: SCIENCE & TECHNOLOGY
THE CONTEXT: The Central Drug Standards Control Organisation (CDSCO) flagged calcium supplement Shelcal 500, antacid Pan D, Urimax D, and Deca-Durabolin 25 Injecti.
EXPLANATION:
More about news:
The Central Drugs Standard Control Organization has recently banned several poor-quality medicines that can have serious health effects, including.
- Shelcal 500: A calcium supplement, often used for bone health, may lead to deficiencies in calcium treatment if ineffective, especially concerning vulnerable groups like the elderly and those with bone health issues.
- Pan D: Used to treat acid reflux and gastrointestinal issues, poor quality could exacerbate symptoms and create complications.
- Urimax D: Commonly prescribed for benign prostatic hyperplasia, its compromised quality may result in treatment failures and prolonged symptoms for patients.
- Deca-Durabolin 25 Injection: As an anabolic steroid, often prescribed to address muscle-wasting conditions, compromised efficacy or purity could lead to severe health risks, particularly given its powerful effects on the body.
About Central Drugs Standard Control Organisation (CDSCO):
- The Central Drugs Standard Control Organisation (CDSCO) has issued a cautionary statement regarding the manufacture and sale of unapproved drugs, particularly emphasising the category of “New Drugs.” It works under the Ministry of Health & Family Welfare.
- It is the National Regulatory Authority (NRA) of India for the medical devices industry under the provisions of the Drugs & Cosmetics Rules.
- Drugs Controller General of India (DCGI) is the head of the CDSCO.
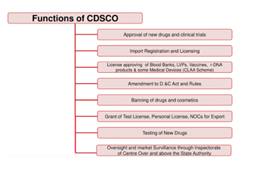
- Under the Drugs and Cosmetics Act, CDSCO is responsible for,
- Approval of New Drugs
- Conduct of Clinical Trials
- Laying down the standards for Drugs
- Control over the quality of imported Drugs in the country
- Coordination of the activities of State Drug Control Organizations
- CDSCO, along with state regulators, is jointly responsible for the grant of licenses for certain specialized categories of critical drugs, such as blood and blood products, I. V. Fluids, Vaccine and Sera.
Drug Controller General of India:
- DCGI heads the Central Drugs Standard Control Organisation (CDSCO), which is responsible for ensuring the quality of drug supply across the country.
- Nodal Ministry: Ministry of Health & Family Welfare
- Functions:
o Give approval to new drugs and regulate clinical trials.
o Sets standards for the manufacturing, sales, import, and distribution of drugs in India.
o Approval of licences of specified categories of drugs such as blood and blood products, IV fluids, vaccines and sera in India.
o Envisages uniform implementation of the provisions of the Drugs & Cosmetics Act, 1940 Act & Rules made there under for ensuring the safety, rights and wellbeing of the patients.
Spread the Word



