TAG: GS-3 SCIENCE AND TECHNOLOGY
THE CONTEXT: The speedy approval of Covid-19 vaccines during the SARS-CoV-2 pandemic highlighted the importance of clinical trials.
EXPLANATION:
What is a Clinical Trial?
- A clinical trial is a research study conducted on human participants to evaluate the safety, efficacy, and/or effectiveness of an intervention such as a drug, device, or behavioural therapy.
- Clinical trials can involve new drugs, devices, procedures, or ways to change behaviors. They can also help improve the quality of life for people with chronic or acute illnesses.
- Amis: Clinical trials are an essential component of the drug development process and are necessary to determine the benefits and risks of new treatments.
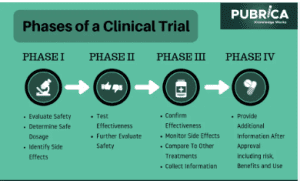
What is CTRI?
The Clinical Trials Registry – India (CTRI) (est. 2007; maintained by ICMR (under the Ministry of Health and Family Welfare) a national clinical trial registry in India.
- It is mandatory to register for every trial at CTRI before commencing
- CTRI is a free online public record system for the registration of clinical trials (both public and private research) conducted in India.
Issues with the CTRI in India:
- Ensure compliance with regulations: The CTRI should ensure registration of all clinical trials in India
- Adhere to WHO guidelines: The CTRI should comply with the WHO guidelines and provide information for each trial, including audit trails, results fields, and data-sharing plans.
- Improve record-keeping of CTRI
- Make CTRI a permanent activity: The CTRI could be made a permanent activity with staff on a five-year contract, instead of relying on temporary staff for 15 years.
Drug approval in India:
- A pharmaceutical company in India must have DCGI approval for selling a new prescription drug. They can get DCGI approval once drug regulator CDSCO verifies the
- quality, safety, and efficacy of drugs including vaccines. Also, required is the approval from respective Ethics Committee where the study is planned and mandatory registration on the ICMR-maintained website
- The Central Drugs Standard Control Organisation (statutory body under the Drugs and Cosmetics Act 1940) is India’s national regulatory body for cosmetics, pharmaceuticals and medical devices.
Source:
Spread the Word



