THE CONTEXT: India is often called the “pharmacy of the world.” Indian pharmaceutical companies supply approximately 40% of generics in the U.S. and 25% in the U.K. and dominate global anti-retroviral drug supplies with a contribution of over 60%. India needs to address challenges related to quality control, regulatory compliance, and competition from other emerging markets.
THE SIGNIFICANCE OF GENERIC DRUGS IN INDIA:
-
- Impact on Out-of-Pocket Healthcare Expenditure: Medicines account for a substantial portion of healthcare costs – 29.1% for inpatient and 60.3% for outpatient out-of-pocket expenditure. Generic medicines are priced 50-90% lower than branded counterparts.
- Pradhan Mantri Bhartiya Janaushadhi Pariyojana: Jan Aushadhi Kendras expanded from 80 in 2014 to over 14,000 by October 2024. The target is to reach 25,000 Kendras in the next two years. Over the past decade, estimated savings of ₹30,000 crore for citizens, and savings reached ₹5,000 crore in FY 2024-25. It has 2047 medicines and 300 surgical devices covering major therapeutic groups, including cardiovascular, anti-cancer, anti-diabetic, and other essential medications.
QUALITY CONCERNS IN GENERIC DRUGS:
-
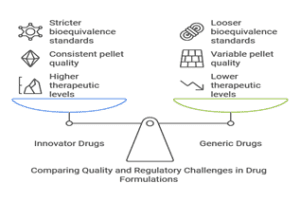
- Comparison between innovator and generic formulations: The Postgraduate Institute of Medical Education and Research (PGIMER) conducted a groundbreaking study comparing the efficacy of generic and innovator itraconazole in treating chronic pulmonary aspergillosis (CPA).
- After two weeks, 73% of subjects achieved therapeutic levels (≥0.5 mg/L) with the innovator drug, compared to only 29% with generic brands. The innovator drug showed higher median trough levels (0.8 mg/L) than generic brands (0 mg/L). Generic brands exhibited variable pellet numbers, sizes, and dummy pellets, indicating quality inconsistencies.
- Excipient variations: Though pharmacologically inactive, excipients play a crucial role in drug formulation and can significantly impact bioavailability. Differences in excipients between generic and innovator drugs may lead to altered dissolution rates, changes in drug stability and variations in drug delivery mechanisms.
- Manufacturing process differences can significantly influence drug quality and efficacy. Key differences include the punching machines used, compression force applied, and granulation methods employed. These variations can affect tablet hardness, particle size, and porosity.
- Bioequivalence threshold limitations: Current regulatory standards for bioequivalence allow for a certain degree of variation between generic and innovator drugs. They allow up to a 20% difference in bioavailability between generic and innovator drugs. However, they may be inadequate for medications with narrow therapeutic indices and drugs requiring precise dosing for optimal efficacy and safety.
- Comparison between innovator and generic formulations: The Postgraduate Institute of Medical Education and Research (PGIMER) conducted a groundbreaking study comparing the efficacy of generic and innovator itraconazole in treating chronic pulmonary aspergillosis (CPA).
REGULATORY CHALLENGES IN INDIA’S GENERIC DRUG INDUSTRY:
-
- Decentralized Drug Regulation System: The Central Drugs Standard Control Organisation (CDSCO), led by the Drugs Controller General of India (DCGI), sets overarching policies and approves new drugs. However, the actual implementation, including licensing, inspections, and local enforcement, is delegated to State Drug Regulatory Authorities (SDRAs).
- Inconsistent enforcement: Varying levels of competence and infrastructure among state regulators result in non-uniform interpretation and implementation of drug laws.
- Regulatory arbitrage: Companies may shift production to states with weaker oversight, compromising drug quality and undermining the reputation of Indian pharmaceuticals in international markets.
- Historical lack of mandatory testing: Until recently, stability testing was only compulsory for ‘patent and proprietary’ drugs, not for generics approved by state licensing authorities. Despite recommendations from the Drugs Consultative Committee in 2013, mandatory stability testing for all generics was not enforced until April 2018.
- Permissive Pharmacopoeia Standards: India’s Pharmacopoeia permits higher drug impurity levels than U.S. and EU standards. The Pharmacopoeia Commission and CDSCO have rejected stricter International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines, citing cost concerns.
- Impurity profiling challenges: The ICH guidelines on impurities in new drug products state that identifying impurities below 0.1% is unnecessary unless potential impurities are expected to be unusually potent or toxic.

| COMMITTEE NAME | YEAR | KEY RECOMMENDATIONS |
| Bhatia Committee | 1954 | Recommended centralization of drug regulatory functions for uniform enforcement. |
| Hathi Committee | 1975 | Advocated for self-reliance in drug production and promotion of generic medicines. |
| Mashelkar Committee | 2003 | Suggested creating a centralized drug regulatory authority and strengthening CDSCO. |
| Drugs Consultative Committee | 2013 | Proposed mandatory stability testing for all generic drugs to ensure efficacy. |
THE WAY FORWARD:
-
- Centralization of Drug Regulation: Establish a National Drug Authority (NDA) as recommended by the Mashelkar Committee (2003). Amend the Drugs and Cosmetics Act, 1940 to transfer key regulatory functions from states to the central authority. Create a single-window system for drug approvals, inspections, and enforcement.
- Strengthening CDSCO and Testing Infrastructure: Increase CDSCO’s budget from the current 0.02% of the health budget to at least 1% by 2025. Establish ten new central drug testing laboratories by 2026, focusing on high-volume generic drugs. Recruit and train 5,000 additional drug inspectors over the next five years. As of 2021, India had only 1,800 drug inspectors against the required 3,200 (WHO recommendation).
- Harmonization of Pharmacopoeia Standards: Align Indian Pharmacopoeia standards with international norms to enhance drug quality and global competitiveness. Adopt ICH Q3A(R2) and Q3B(R2) guidelines for impurity profiling in active pharmaceutical ingredients and finished products. Implement a phased approach to tighten impurity limits, reaching parity with USP and EP standards by 2028.
- Comprehensive Stability Testing Framework: Mandate stability testing for all generic drugs, including those approved before 2018. Implement WHO guidelines on stability testing of pharmaceutical products in various climatic zones. Establish a national stability testing database to track and analyze long-term drug stability data.
- Bioequivalence and Therapeutic Equivalence Enhancement: Revise the bioequivalence acceptance criteria for narrow therapeutic index drugs from 80-125% to 90-111%. Implement in vivo bioequivalence studies for BCS Class II and IV drugs. Introduce mandatory therapeutic equivalence studies for selected drug classes (e.g., antiepileptics, immunosuppressants).
- Capacity Building and Research Promotion: Establish a National Institute of Pharmaceutical Education and Research (NIPER) in each state by 2030. Allocate 2% of the pharmaceutical industry’s turnover to a research fund for generic drug improvement. Implement a “Pharma Vision 2030” program to train 100,000 skilled professionals in advanced pharmaceutical technologies.
THE CONCLUSION:
India must centralize drug regulation, strengthen CDSCO, and harmonize pharmacopeia standards to enhance generic drug quality and maintain its global leadership. With a projected pharmaceutical market value of $130 billion by 2030 and the potential to save ₹60,000 crore annually through quality generic medicines, these reforms are crucial for ensuring affordable, safe, and effective healthcare for all Indians.
UPSC PAST YEAR QUESTION:
Q. Can overuse and the availability of antibiotics without a doctor’s prescription contribute to the emergence of drug-resistant diseases in India? What are the available mechanisms for monitoring and control? Critically discuss the various issues involved. 2014
MAINS PRACTICE QUESTION:
Q. India’s generic drug industry plays a crucial role in global healthcare but faces significant quality and regulatory challenges. Critically examine
SOURCE:
https://www.thehindu.com/opinion/lead/making-affordable-generics-more-reliable/article69000243.ece
Spread the Word
