Day-747
Quiz-summary
0 of 20 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
Information
DAILY MCQ
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Results
0 of 20 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 points, (0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- Answered
- Review
-
Question 1 of 20
1. Question
1. Consider the following statements:
1. Vibration is a type of periodic motion.
2. Rotational motion occurs around a defined axis.
3. Rolling includes rotatory motion.
How many of the above statements are correct?Correct
Answer: C
Explanation:
• Statement 1 is CORRECT: Vibration is a to and fro motion which is periodic in nature. In vibration the object moves quickly between mean and extreme positions.
• Statement 2 is CORRECT: Rotational motion is the movement of a body around a fixed axis.
• Statement 3 is CORRECT: Rolling is a combination of rotational and translational motion of a round shaped body. Eg- rolling of car’s wheel on road is a type of rolling.
Various types of motions
1. Rectilinear motion: Motion along a straight-line path. Eg – Running on straight road.
2. Curvilinear motion: Motion along a curved path. Eg – Moving on a curved road.
3. Circulatory motion: Motion along a circular or closed path. Eg – Running on a circular track.
4. Rotational motion: Motion along a fixed axis. Eg – Rotating wheel
5. Periodic motion: Motion which keeps repeating itself after a fixed interval of time. Eg – Motion of a bob of a pendulum.
Note: Oscillatory motion and vibratory motion are types of Periodic motion.
Source: NCERT Class 11th PhysicsIncorrect
Answer: C
Explanation:
• Statement 1 is CORRECT: Vibration is a to and fro motion which is periodic in nature. In vibration the object moves quickly between mean and extreme positions.
• Statement 2 is CORRECT: Rotational motion is the movement of a body around a fixed axis.
• Statement 3 is CORRECT: Rolling is a combination of rotational and translational motion of a round shaped body. Eg- rolling of car’s wheel on road is a type of rolling.
Various types of motions
1. Rectilinear motion: Motion along a straight-line path. Eg – Running on straight road.
2. Curvilinear motion: Motion along a curved path. Eg – Moving on a curved road.
3. Circulatory motion: Motion along a circular or closed path. Eg – Running on a circular track.
4. Rotational motion: Motion along a fixed axis. Eg – Rotating wheel
5. Periodic motion: Motion which keeps repeating itself after a fixed interval of time. Eg – Motion of a bob of a pendulum.
Note: Oscillatory motion and vibratory motion are types of Periodic motion.
Source: NCERT Class 11th Physics -
Question 2 of 20
2. Question
2. Consider the following cases:
1. A man pushing a wall
2. Running on a treadmill
3. Walking on an inclined path
4. A lady cooking food.
How many of the above cases constitute work done?Correct
Answer: A
Explanation:
• Case 1 is INCORRECT: A man pushing a wall does no work as the wall is not moving or displacing from its place
• Case 2 is INCORRECT: Running on a treadmill also does not constitute a work since there is no displacement of the person running on a treadmill.
• Case 3 is CORRECT: Walking on an inclined path is work done against gravity since the movement is against the force of gravity.
• Case 4 is INCORRECT: A lady cooking food is not a work in physics since there is neither any force being applied nor is there any displacement.
Source: NCERT Class 11th PhysicsIncorrect
Answer: A
Explanation:
• Case 1 is INCORRECT: A man pushing a wall does no work as the wall is not moving or displacing from its place
• Case 2 is INCORRECT: Running on a treadmill also does not constitute a work since there is no displacement of the person running on a treadmill.
• Case 3 is CORRECT: Walking on an inclined path is work done against gravity since the movement is against the force of gravity.
• Case 4 is INCORRECT: A lady cooking food is not a work in physics since there is neither any force being applied nor is there any displacement.
Source: NCERT Class 11th Physics -
Question 3 of 20
3. Question
3. Which of the following cases holds true when a ball is being dropped from a height of 20 m above the ground?
Correct
Answer: C
Explanation:
• When the ball is at the highest point, its potential energy is maximum but kinetic energy is zero.
• When the ball is just above the ground, its kinetic energy is maximum but potential energy is near zero.
• During the fall it is evident that the potential energy decreases, and the kinetic energy increases during the fall.
• The above situation is governed by the law of conservation of energy.
Source: NCERT Class 11th PhysicsIncorrect
Answer: C
Explanation:
• When the ball is at the highest point, its potential energy is maximum but kinetic energy is zero.
• When the ball is just above the ground, its kinetic energy is maximum but potential energy is near zero.
• During the fall it is evident that the potential energy decreases, and the kinetic energy increases during the fall.
• The above situation is governed by the law of conservation of energy.
Source: NCERT Class 11th Physics -
Question 4 of 20
4. Question
4. With reference to SI units of physical quantities, consider the following pairs:
Physical Quantity : Unit
1. Work : Newton
2. Power : Watt
3. Energy : Joule
How many of the above pairs are correctly matched?Correct
Answer: B
Explanation:
• Pair 1 is INCORRECT: The SI unit of Work is Joule.
• Pair 2 is CORRECT: The SI unit of Power is Watt which is equal to Newton metre per second.
• Pair 3 is CORRECT: The SI unit of energy is Joule. SI unit of Work as well as energy is Joule which is equal to Newton metre.
Source: NCERT Class 11th PhysicsIncorrect
Answer: B
Explanation:
• Pair 1 is INCORRECT: The SI unit of Work is Joule.
• Pair 2 is CORRECT: The SI unit of Power is Watt which is equal to Newton metre per second.
• Pair 3 is CORRECT: The SI unit of energy is Joule. SI unit of Work as well as energy is Joule which is equal to Newton metre.
Source: NCERT Class 11th Physics -
Question 5 of 20
5. Question
5. Consider the following statements:
1. Power is defined as the capacity to do work.
2. More power means more work can be done in less period of time.
3. Electrical power can be converted into mechanical power without any loss of power.
How many of the above statements are correct?Correct
Answer: A
Explanation:
• Statement 1 is INCORRECT: Energy is defined as the capacity to do work.
• Statement 2 is CORRECT: More power means more work in less period of time as power is defined as rate of work done.
• Statement 3 is INCORRECT: There is no machine or mechanism that can convert one form of power to another without any loss in power.
Source: NCERT Class 11th PhysicsIncorrect
Answer: A
Explanation:
• Statement 1 is INCORRECT: Energy is defined as the capacity to do work.
• Statement 2 is CORRECT: More power means more work in less period of time as power is defined as rate of work done.
• Statement 3 is INCORRECT: There is no machine or mechanism that can convert one form of power to another without any loss in power.
Source: NCERT Class 11th Physics -
Question 6 of 20
6. Question
Consider the following statements:
1. Mass of proton and electron constitute the Atomic Mass of an element.
2. Number of protons in an element constitute the Atomic Number of the element.
3. Electrons are heavier than protons in any atom.
How many of the above statements are correct?Correct
Answer: A
Explanation:
In any atom, there are there subatomic particles – Neutrons, Protons and Electrons. Neutrons and Protons together make up the nucleus of any atom whereas electrons revolve around the nucleus in orbits. However, it is to be noted that we cannot locate the electrons precisely and thus a probability function is generated which makes up the electron cloud of any atom.
• Statement 1 is INCORRECT: Atomic mass is the total mass of one atom of the given element. It is denoted by the symbol ‘u’. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Mass number is the total number of protons and neutrons in any atom of an element.
• Statement 2 is CORRECT: The Atomic Number is the number of protons in the nucleus of an atom. The number of protons defines the identity of an element. The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behaviour of an element.
• Statement 3 is INCORRECT: In any atom, electrons are the lightest sub atomic particle out of proton, neutron and electron. Infact, Protons are approximately 2000 times heavier than electrons in an atom.
Source: NCERT class 11th ChemistryIncorrect
Answer: A
Explanation:
In any atom, there are there subatomic particles – Neutrons, Protons and Electrons. Neutrons and Protons together make up the nucleus of any atom whereas electrons revolve around the nucleus in orbits. However, it is to be noted that we cannot locate the electrons precisely and thus a probability function is generated which makes up the electron cloud of any atom.
• Statement 1 is INCORRECT: Atomic mass is the total mass of one atom of the given element. It is denoted by the symbol ‘u’. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Mass number is the total number of protons and neutrons in any atom of an element.
• Statement 2 is CORRECT: The Atomic Number is the number of protons in the nucleus of an atom. The number of protons defines the identity of an element. The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behaviour of an element.
• Statement 3 is INCORRECT: In any atom, electrons are the lightest sub atomic particle out of proton, neutron and electron. Infact, Protons are approximately 2000 times heavier than electrons in an atom.
Source: NCERT class 11th Chemistry -
Question 7 of 20
7. Question
7. Consider the following pairs:
Particle – Discovered by
1. Electron – Ernest Rutherford
2. Proton – JJ Thomson
3. Neutron – James Chadwick
How many of the above pairs are correctly matched?Correct
Answer: A
• Pair 1 is INCORRECT: Electrons were discovered by J.J. Thomson in 1897 when he was studying the properties of cathode ray and won the Noble Prize in 1906 for discovering Electrons. Thomson proposed that an atom consists of a positively charged sphere and the electrons are embedded in it.
• Pair 2 is INCORRECT: Protons were discovered by Goldstein. In 1886, Eugen Goldstein discovered canal rays or positive rays. Rutherford gave model of the atom by performing an experiment with thin gold foil and positively charged alpha particles.
• Pair 3 is CORRECT: Neutrons were discovered by James Chadwick. He bombarded a thin sheet of Beryllium (Be) by alpha particles, electrically neutral particles having a mass slightly greater than that of protons i.e. neutrons were emitted.
Source: Class 9th Science NCERTIncorrect
Answer: A
• Pair 1 is INCORRECT: Electrons were discovered by J.J. Thomson in 1897 when he was studying the properties of cathode ray and won the Noble Prize in 1906 for discovering Electrons. Thomson proposed that an atom consists of a positively charged sphere and the electrons are embedded in it.
• Pair 2 is INCORRECT: Protons were discovered by Goldstein. In 1886, Eugen Goldstein discovered canal rays or positive rays. Rutherford gave model of the atom by performing an experiment with thin gold foil and positively charged alpha particles.
• Pair 3 is CORRECT: Neutrons were discovered by James Chadwick. He bombarded a thin sheet of Beryllium (Be) by alpha particles, electrically neutral particles having a mass slightly greater than that of protons i.e. neutrons were emitted.
Source: Class 9th Science NCERT -
Question 8 of 20
8. Question
8. Consider the following pairs:
Name – Explanation
1. Isotone – Atoms having same number of Neutrons
2. Isotope – Atoms having same Atomic Number but different Mass Number
3. Isobar – Atoms having same Mass Number but different Atomic Number
How many of the above pairs are correctly matched?Correct
Answer: C
Explanation:
Atomic Number: Number of Protons in any atom.
Mass Number: Sum of Number of Protons and Neutrons in any atom.
• Pair 1 is CORRECT: Atoms with the same number of protons but different numbers of neutrons are called isotopes. Since, the number of Neutrons differs so does the Mass Number. They share almost the same chemical properties, but differ in mass and therefore in physical properties.
• Pair 2 is CORRECT: Isotones are atoms that have the same neutron number but different proton number.
• Pair 3 is CORRECT: Isobars are elements that have the same number of nucleons (sum of protons and neutrons). The number of nucleons is also known as Mass Number.
Source: NCERT Class 9th ScienceIncorrect
Answer: C
Explanation:
Atomic Number: Number of Protons in any atom.
Mass Number: Sum of Number of Protons and Neutrons in any atom.
• Pair 1 is CORRECT: Atoms with the same number of protons but different numbers of neutrons are called isotopes. Since, the number of Neutrons differs so does the Mass Number. They share almost the same chemical properties, but differ in mass and therefore in physical properties.
• Pair 2 is CORRECT: Isotones are atoms that have the same neutron number but different proton number.
• Pair 3 is CORRECT: Isobars are elements that have the same number of nucleons (sum of protons and neutrons). The number of nucleons is also known as Mass Number.
Source: NCERT Class 9th Science -
Question 9 of 20
9. Question
9. Consider the following statements:
Statement I: Aluminium is more resistant to corrosion than iron.
Statement II: Aluminium forms a thin layer of aluminium oxide but iron forms flakes of iron oxide when exposed to air or water.
Which one of the following is correct in respect of the above statements?Correct
Answer: A
Explanation:
Corrosion is the gradual decomposition and deterioration of metals due to oxidation when exposed to air or water. It is a chemical process and is naturally occurring. Rusting of iron, or the forming of a brown flaky material on iron objects when exposed to moist air, is the most common example of metal corrosion. When corrosion has been discovered, the only sure way to repair it is to remove it. Abrasion, followed by a corrosion inhibitor, such as zinc-chromate primer, another primer, and finally paint, will eliminate light surface corrosion.
• Both Statements are CORRECT and Statement 2 is the CORRECT explanation of Statement 1
• Statement 1 is CORRECT: Aluminium is more resistant to corrosion than iron because it forms a thin layer of aluminium oxide which protects the bulk of metal from further corrosion.
• Statement 2 is CORRECT and is the correct explanation of statement 1: Since iron corrodes by formation of flakes, thus every time the raw metal is exposed to oxidation. This damages the iron made object till it is fully decomposed. But aluminium forms a resistant layer of its oxide which prevents further corrosion of the bulk of the metal.
Source: NCERT class 10th ScienceIncorrect
Answer: A
Explanation:
Corrosion is the gradual decomposition and deterioration of metals due to oxidation when exposed to air or water. It is a chemical process and is naturally occurring. Rusting of iron, or the forming of a brown flaky material on iron objects when exposed to moist air, is the most common example of metal corrosion. When corrosion has been discovered, the only sure way to repair it is to remove it. Abrasion, followed by a corrosion inhibitor, such as zinc-chromate primer, another primer, and finally paint, will eliminate light surface corrosion.
• Both Statements are CORRECT and Statement 2 is the CORRECT explanation of Statement 1
• Statement 1 is CORRECT: Aluminium is more resistant to corrosion than iron because it forms a thin layer of aluminium oxide which protects the bulk of metal from further corrosion.
• Statement 2 is CORRECT and is the correct explanation of statement 1: Since iron corrodes by formation of flakes, thus every time the raw metal is exposed to oxidation. This damages the iron made object till it is fully decomposed. But aluminium forms a resistant layer of its oxide which prevents further corrosion of the bulk of the metal.
Source: NCERT class 10th Science -
Question 10 of 20
10. Question
10. With reference to the applications of base, consider the following examples:
1. Neutralisation of soil which is high in organic matter.
2. Cleaning of metals before electroplating.
3. Manufacturing of soaps.
How many of the above examples are correct?Correct
Answer: B
Explanation:
A base is a substance that can neutralize the acid by producing hydroxide ions. Neutralisation is a reaction of base with acids to form water and salts. Bases include the oxides, hydroxides and carbonates of metals. The soluble bases are called alkalis. They are bitter in taste.
• Example 1 is CORRECT: High organic matter in soil leads to acidic nature of soil due to the formation of humic acid. Bases like lime is added to neutralise the effects of high levels of soil acidity.
• Example 2 is INCORRECT: Acids like Hydrochloric acid is used to clean metals before electroplating since acid reacts with the metal oxide which is formed because of corrosion. A base does not generally react with a metal oxide.
• Example 3 is CORRECT: Alkali and fat is used in a process called as saponification to produce soaps. Bases like Caustic soda (Sodium Hydroxide) is used in the manufacturing of soaps and detergents.
Source: NCERT class 10th ScienceIncorrect
Answer: B
Explanation:
A base is a substance that can neutralize the acid by producing hydroxide ions. Neutralisation is a reaction of base with acids to form water and salts. Bases include the oxides, hydroxides and carbonates of metals. The soluble bases are called alkalis. They are bitter in taste.
• Example 1 is CORRECT: High organic matter in soil leads to acidic nature of soil due to the formation of humic acid. Bases like lime is added to neutralise the effects of high levels of soil acidity.
• Example 2 is INCORRECT: Acids like Hydrochloric acid is used to clean metals before electroplating since acid reacts with the metal oxide which is formed because of corrosion. A base does not generally react with a metal oxide.
• Example 3 is CORRECT: Alkali and fat is used in a process called as saponification to produce soaps. Bases like Caustic soda (Sodium Hydroxide) is used in the manufacturing of soaps and detergents.
Source: NCERT class 10th Science -
Question 11 of 20
11. Question
11. With reference to the gravitational Force between two bodies, consider the following statements:
1. Force of gravitation depends upon the masses of the two interacting bodies.
2. Force of gravitation is independent of the distance between the two bodies.
3. Gravitational force is one of the four fundamental forces of nature.
How many of the above statements are correct?Correct
Answer: B
Explanation:
• Statement 1 is CORRECT: Force of gravitation is directly proportional to the mass of two interacting bodies.
• Statement 2 is INCORRECT: Force of gravitation is indirectly proportional to the square of the distance between two bodies under consideration.
• Statement 3 is CORRECT: Gravitational force is one of the four fundamental forces of nature.
Fundamental forces of nature.
a. Strong Force
b. Weak Force
c. Gravitational force
d. ElectromagnetismSource: NCERT Class 11th Physics
Incorrect
Answer: B
Explanation:
• Statement 1 is CORRECT: Force of gravitation is directly proportional to the mass of two interacting bodies.
• Statement 2 is INCORRECT: Force of gravitation is indirectly proportional to the square of the distance between two bodies under consideration.
• Statement 3 is CORRECT: Gravitational force is one of the four fundamental forces of nature.
Fundamental forces of nature.
a. Strong Force
b. Weak Force
c. Gravitational force
d. ElectromagnetismSource: NCERT Class 11th Physics
-
Question 12 of 20
12. Question
12. Consider the following statements:
1. A satellite revolves around the planet by escaping the gravitational field of the planet.
2. Satellites maintain orbital stability through high speed of revolution.
3. The Moon orbits around the Earth due to gravitational force acting between the Earth and the Moon.
How many of the above statements are correct?Correct
Answer: B
Explanation:
• Statement 1 is INCORRECT: Satellites do not escape the gravitational field of the planet around which they revolve. If they escape the field, then they may deviate from the orbit and start wandering in the universe like a lost celestial body.
• Statement 2 is CORRECT: The high speed of revolution of satellites (or orbital velocity) helps them to overcome the gravitational force of attraction so that they maintain orbit stability. The lower the orbital radius, the greater the orbital velocity.
• Statement 3 is CORRECT: Moon orbits around the Earth due to gravitational force acting between the two bodies.
Source: NCERT Class 11th PhysicsIncorrect
Answer: B
Explanation:
• Statement 1 is INCORRECT: Satellites do not escape the gravitational field of the planet around which they revolve. If they escape the field, then they may deviate from the orbit and start wandering in the universe like a lost celestial body.
• Statement 2 is CORRECT: The high speed of revolution of satellites (or orbital velocity) helps them to overcome the gravitational force of attraction so that they maintain orbit stability. The lower the orbital radius, the greater the orbital velocity.
• Statement 3 is CORRECT: Moon orbits around the Earth due to gravitational force acting between the two bodies.
Source: NCERT Class 11th Physics -
Question 13 of 20
13. Question
13. Consider the following examples:
1. Missile launched from fighter jet.
2. Racing Car running on a track.
3. Swinging of a cricket ball.
How many of the above examples show the applications of Bernoulli’s Principle?Correct
Answer: C
Explanation:
Bernoulli’s Principle:
Bernoulli’s principle states that an increase in the speed of a fluid occurs simultaneously with a decrease in static pressure or the fluid’s potential energy.
• Example 1 is CORRECT: Fluid flows from high pressure at the head end on the left towards the low pressure at the exhaust on the right. Thus, missiles generate thrust to propel themselves to very high speeds.
• Example 2 is CORRECT: A race car employs Bernoulli’s principle to keep its rear wheels on the ground at all times while travelling at high speeds. A race car’s spoiler is shaped like an upside-down wing, with the curved surface at the bottom. This produces a net downward force, thus keeping the car to the ground.
• Example 3 is CORRECT: One side of the ball is rough which reduces speed of air flowing. This increases pressure on that side. Smooth side has lower pressure. This creates pressure difference which causes swing in a cricket ball.
Source: https://www.princeton.edu/~asmits/Bicycle_web/Bernoulli.htmlIncorrect
Answer: C
Explanation:
Bernoulli’s Principle:
Bernoulli’s principle states that an increase in the speed of a fluid occurs simultaneously with a decrease in static pressure or the fluid’s potential energy.
• Example 1 is CORRECT: Fluid flows from high pressure at the head end on the left towards the low pressure at the exhaust on the right. Thus, missiles generate thrust to propel themselves to very high speeds.
• Example 2 is CORRECT: A race car employs Bernoulli’s principle to keep its rear wheels on the ground at all times while travelling at high speeds. A race car’s spoiler is shaped like an upside-down wing, with the curved surface at the bottom. This produces a net downward force, thus keeping the car to the ground.
• Example 3 is CORRECT: One side of the ball is rough which reduces speed of air flowing. This increases pressure on that side. Smooth side has lower pressure. This creates pressure difference which causes swing in a cricket ball.
Source: https://www.princeton.edu/~asmits/Bicycle_web/Bernoulli.html -
Question 14 of 20
14. Question
14. Consider the following statements:
Statement I: Air Conditioners (ACs) consume electricity to pump the refrigerant for cooling.
Statement II: ACs use electricity to transfer heat from lower temperature to higher temperature, which is not spontaneous.
Which one of the following is correct in respect of the above statements?Correct
Answer: B
Explanation:
• Both Statements are CORRECT and Statement 2 is NOT the CORRECT explanation of Statement 1
• Statement 1 is CORRECT: Evaporator coil which is present in the indoor unit of ACs run liquid refrigerant. This refrigerant absorbs heat and changes into vapour form. Thus, electricity is used to compress the gas to condense it into liquid form.
• Statement 2 is CORRECT and is NOT the correct explanation of Statement 1: Spontaneous heat flows from higher temperature to lower temperature. But to transfer heat from lower temperature to higher temperature, additional work must be done. This is according to 2nd law of thermodynamics.Incorrect
Answer: B
Explanation:
• Both Statements are CORRECT and Statement 2 is NOT the CORRECT explanation of Statement 1
• Statement 1 is CORRECT: Evaporator coil which is present in the indoor unit of ACs run liquid refrigerant. This refrigerant absorbs heat and changes into vapour form. Thus, electricity is used to compress the gas to condense it into liquid form.
• Statement 2 is CORRECT and is NOT the correct explanation of Statement 1: Spontaneous heat flows from higher temperature to lower temperature. But to transfer heat from lower temperature to higher temperature, additional work must be done. This is according to 2nd law of thermodynamics. -
Question 15 of 20
15. Question
15. Consider the following:
1. Length of Pendulum
2. Mass of the oscillating bob
3. Acceleration due to gravity
The time period of a simple pendulum depends upon how many of the above physical quantities?Correct
Answer: B
Explanation:

A simple pendulum is the most common example of bodies executing Simple Harmonic Motion (S.H.M.) An ideal simple pendulum consists of a heavy point mass body suspended by a weightless in extensible and perfectly flexible string from a rigid support about which it is free to oscillate.
The time period of a simple pendulum depends on
(i) length of the pendulum and
(ii) the acceleration due to gravity (g).
Time period (T) = 2π√(l/g)
Source: NCERT class 11th PhysicsIncorrect
Answer: B
Explanation:

A simple pendulum is the most common example of bodies executing Simple Harmonic Motion (S.H.M.) An ideal simple pendulum consists of a heavy point mass body suspended by a weightless in extensible and perfectly flexible string from a rigid support about which it is free to oscillate.
The time period of a simple pendulum depends on
(i) length of the pendulum and
(ii) the acceleration due to gravity (g).
Time period (T) = 2π√(l/g)
Source: NCERT class 11th Physics -
Question 16 of 20
16. Question
16. Consider the following statements:
1. While diluting acid, it is safer to add acid into water than water into acid.
2. Gases of acid is more hazardous than liquid form of acid.
Which of the statements given above is/are correct?Correct
Answer: A
Explanation:
• Statement 1 is CORRECT: Mixing of water and acid is a highly exothermic reaction which means that a high amount of heat is released. While we dilute acid, it is always preferred that the acid is added to water rather than the water being added to the acid. As adding water to a concentrated acid releases a huge amount of heat, which can cause an explosion and acid burns on the skin, clothing, and other body parts. This is because the heat released in case of mixing of acid with water can be evenly distributed in case of large quantity of water. This is possible only when acid is added drop by drop in water than the other way round.
• Statement 2 is INCORRECT: Gaseous form of acid does not release the hydrogen ion. It is only released when acid is in form of aqueous form (solution of water). Acidic action can only be initiated in case of release of hydrogen ion. However, it is to be noted that inhaling dry acid gas or exposure to eyes can be dangerous as the water in the body or the eyes can rapidly release heat and hydrogen ion formation which can even cause death or blindness.
Source: NCERT class 10th ScienceIncorrect
Answer: A
Explanation:
• Statement 1 is CORRECT: Mixing of water and acid is a highly exothermic reaction which means that a high amount of heat is released. While we dilute acid, it is always preferred that the acid is added to water rather than the water being added to the acid. As adding water to a concentrated acid releases a huge amount of heat, which can cause an explosion and acid burns on the skin, clothing, and other body parts. This is because the heat released in case of mixing of acid with water can be evenly distributed in case of large quantity of water. This is possible only when acid is added drop by drop in water than the other way round.
• Statement 2 is INCORRECT: Gaseous form of acid does not release the hydrogen ion. It is only released when acid is in form of aqueous form (solution of water). Acidic action can only be initiated in case of release of hydrogen ion. However, it is to be noted that inhaling dry acid gas or exposure to eyes can be dangerous as the water in the body or the eyes can rapidly release heat and hydrogen ion formation which can even cause death or blindness.
Source: NCERT class 10th Science -
Question 17 of 20
17. Question
17. Consider the following statements:
1. Bleaching powder is used as a reducing agent in many chemical industries.
2. Washing soda is also used in soda-acid fire extinguisher.
3. On heating Plaster of Paris at 100 degrees Celsius, it becomes Gypsum.
How many of the above statements are correct?Correct
Answer: D
Explanation:
• Statement 1 is INCORRECT: Bleaching powder is a commonly used chemical compound that is used for a variety of purposes such as bleaching, disinfecting, and water treatment. It is also used as an oxidizing agent in various chemical reactions. Oxidation is a chemical reaction in which a substance loses electrons. An oxidizing agent is a substance that accepts electrons from another substance and gets reduced in the process.
• Statement 2 is INCORRECT: Baking soda is used in soda acid fire extinguisher. This is because when the sulphuric acid reacts with the baking soda, it releases carbon dioxide. This extinguishes the flame as supply of oxygen is cut. Baking soda is also known as sodium bicarbonate.
• Statement 3 is INCORRECT: On heating gypsum at 100 degrees Celsius, it becomes Plaster of Paris as some molecules of water is lost. Gypsum is used in cement industry, whereas Plaster of Paris is used as plastering fractures. When water is again added to Plaster of Paris, it again gets converted into gypsum, giving a hard solid mass.
Source: NCERT class 10th ScienceIncorrect
Answer: D
Explanation:
• Statement 1 is INCORRECT: Bleaching powder is a commonly used chemical compound that is used for a variety of purposes such as bleaching, disinfecting, and water treatment. It is also used as an oxidizing agent in various chemical reactions. Oxidation is a chemical reaction in which a substance loses electrons. An oxidizing agent is a substance that accepts electrons from another substance and gets reduced in the process.
• Statement 2 is INCORRECT: Baking soda is used in soda acid fire extinguisher. This is because when the sulphuric acid reacts with the baking soda, it releases carbon dioxide. This extinguishes the flame as supply of oxygen is cut. Baking soda is also known as sodium bicarbonate.
• Statement 3 is INCORRECT: On heating gypsum at 100 degrees Celsius, it becomes Plaster of Paris as some molecules of water is lost. Gypsum is used in cement industry, whereas Plaster of Paris is used as plastering fractures. When water is again added to Plaster of Paris, it again gets converted into gypsum, giving a hard solid mass.
Source: NCERT class 10th Science -
Question 18 of 20
18. Question
18. With reference to the occurrence of metals, consider the following statements:
1. Most reactive metals are found in their native states.
2. Noble metals require electrolysis for extraction.
Which of the statements given above is/are NOT correct?Correct
Answer: C
Explanation:
The elements or compounds which occur naturally in the earth’s crust. At some places, these minerals contain a very high percentage of a particular metal which makes extraction economically viable. These minerals are known as ores. Metals are extracted from ores depending on the reactivity of the respective metal.

• Statement 1 is INCORRECT: Most reactive metals like sodium and potassium form a very strong ionic bond with other elements like chlorine and oxygen. They need electrolysis, which is the use of electricity to break the compound into constituent elements. Least reactive metals like gold, silver and platinum are generally found in their native states.
• Statement 2 is INCORRECT: Noble metals like Gold and Platinum are least reactive, so they are found in their native states.
Source: NCERT Class 10th ScienceIncorrect
Answer: C
Explanation:
The elements or compounds which occur naturally in the earth’s crust. At some places, these minerals contain a very high percentage of a particular metal which makes extraction economically viable. These minerals are known as ores. Metals are extracted from ores depending on the reactivity of the respective metal.



• Statement 1 is INCORRECT: Most reactive metals like sodium and potassium form a very strong ionic bond with other elements like chlorine and oxygen. They need electrolysis, which is the use of electricity to break the compound into constituent elements. Least reactive metals like gold, silver and platinum are generally found in their native states.
• Statement 2 is INCORRECT: Noble metals like Gold and Platinum are least reactive, so they are found in their native states.
Source: NCERT Class 10th Science -
Question 19 of 20
19. Question
19. With reference to Alloys, consider the following pairs:
Alloy – Metals
1. Bronze – Copper and Zinc
2. Brass – Copper and Tin
3. Solder – Lead and Tin
How many of the above pairs are correctly matched?Correct
Answer: A
Explanation:
Alloys are made by mixing two or more elements, at least one of which is a metal and the one which is in larger quantity is the base metal. It is a physical mixture in which the physical properties, like hardness, shine, durability etc, of a base metal is enhanced by adding either other metals or non-metals.
• Pair 1 is INCORRECT: Bronze is an alloy of Copper and Tin. Due to its corrosion resistance and unique colouring, bronze is commonly used in the manufacture of coins, medals, hardware mounts, furniture trim, ceiling or wall panels, ship hardware, and all sorts of automobile parts.
• Pair 2 is INCORRECT: Brass is an alloy of Copper and Zinc. Brass is used to manufacture musical instruments like- trumpets, horns etc.
• Pair 3 is CORRECT: Solder is an alloy of Lead and Tin. Solder is used to bond metal workpieces together. Solder is commonly used in electronics, heating, air conditioning, mechanical, fire sprinkler etc.
Source: NCERT Chemistry class 12thIncorrect
Answer: A
Explanation:
Alloys are made by mixing two or more elements, at least one of which is a metal and the one which is in larger quantity is the base metal. It is a physical mixture in which the physical properties, like hardness, shine, durability etc, of a base metal is enhanced by adding either other metals or non-metals.
• Pair 1 is INCORRECT: Bronze is an alloy of Copper and Tin. Due to its corrosion resistance and unique colouring, bronze is commonly used in the manufacture of coins, medals, hardware mounts, furniture trim, ceiling or wall panels, ship hardware, and all sorts of automobile parts.
• Pair 2 is INCORRECT: Brass is an alloy of Copper and Zinc. Brass is used to manufacture musical instruments like- trumpets, horns etc.
• Pair 3 is CORRECT: Solder is an alloy of Lead and Tin. Solder is used to bond metal workpieces together. Solder is commonly used in electronics, heating, air conditioning, mechanical, fire sprinkler etc.
Source: NCERT Chemistry class 12th -
Question 20 of 20
20. Question
20. Consider the following statements:
Statement I: Soaps are not effective in hard water whereas detergents are effective in hard water.
Statement II: Soaps are completely biodegradable whereas detergents are not.
Which one of the following is correct in respect of the above statements?Correct
Answer: B
Explanation:
Soaps are generally prepared via the saponification of fats and oils. They have two ends having different properties -one is hydrophilic which interacts with water, while the other end is hydrophobic, that is, it interacts with oils or dirt. This formation is called as Micelle. Detergents overcome some limitations of soaps due to their chemical compositions.


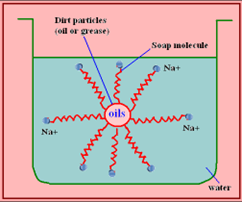
• Both Statements are CORRECT and Statement 2 is NOT the CORRECT explanation of Statement 1.
• Statement 1 is CORRECT: Soaps are not effective in hard water. Hard water contains calcium and magnesium ions. When soaps are dissolved in hard water, these ions displace sodium or potassium from their salts and form insoluble calcium or magnesium salts of fatty acids. These insoluble salts separate as scum. This is the reason why do not work in hard water. Detergents are generally ammonium or sulphonate salts of long chain carboxylic acids. They do not make scum in hard water as these chains do not react with metal ions to form insoluble precipitate.
• Statement 2 is CORRECT but NOT the CORRECT explanation of Statement 1: Soap molecules have straight hydrocarbon chains which are easily degraded by bacteria present in the sewage water. On the other hand, detergent molecules have branched hydrocarbon chains which are either not attacked or slowly attacked by bacteria present in sewage water.
Source: NCERT class 10th ScienceIncorrect
Answer: B
Explanation:
Soaps are generally prepared via the saponification of fats and oils. They have two ends having different properties -one is hydrophilic which interacts with water, while the other end is hydrophobic, that is, it interacts with oils or dirt. This formation is called as Micelle. Detergents overcome some limitations of soaps due to their chemical compositions.



• Both Statements are CORRECT and Statement 2 is NOT the CORRECT explanation of Statement 1.
• Statement 1 is CORRECT: Soaps are not effective in hard water. Hard water contains calcium and magnesium ions. When soaps are dissolved in hard water, these ions displace sodium or potassium from their salts and form insoluble calcium or magnesium salts of fatty acids. These insoluble salts separate as scum. This is the reason why do not work in hard water. Detergents are generally ammonium or sulphonate salts of long chain carboxylic acids. They do not make scum in hard water as these chains do not react with metal ions to form insoluble precipitate.
• Statement 2 is CORRECT but NOT the CORRECT explanation of Statement 1: Soap molecules have straight hydrocarbon chains which are easily degraded by bacteria present in the sewage water. On the other hand, detergent molecules have branched hydrocarbon chains which are either not attacked or slowly attacked by bacteria present in sewage water.
Source: NCERT class 10th Science




