TAG: GS 3: SCIENCE AND TECHNOLOGY
THE CONTEXT: A team of biochemists at the University of Washington has made a significant advancement in the field of protein engineering by developing proteins capable of transitioning between assembly and disassembly through allosteric control.
EXPLANATION:
- This innovative approach, detailed in the journal Nature, represents a major milestone in protein design, offering potential applications in drug delivery, molecular machines, and synthetic biology.
The Significance of Allosteric Control in Protein Engineering
- The concept of manipulating proteins to assemble or disassemble on command has been a long-standing objective in biochemistry.
- Achieving this capability would allow scientists to create highly specific molecular structures that can perform precise functions within biological systems.
- For example, designing a protein that assembles into a cage-like structure could enable targeted drug delivery, where the protein cage transports a therapeutic agent to a specific location within the body.
- Allosteric regulation is a process where a protein’s function is controlled by the binding of a specific molecule (referred to as an effector).
- It has been identified as a key mechanism to achieve this level of control.
- The ability to design proteins that respond to allosteric triggers could revolutionize the way scientists approach the construction of dynamic, functional proteins.
Techniques Used in the Engineering Process
- To achieve their goal, the research team employed a combination of cutting-edge techniques, many of which were developed in their prior work.
- One of the most critical tools in their arsenal was an AI application capable of predicting protein structures based on a given set of attributes.
- This AI-driven approach allowed the team to design proteins with specific features that would be necessary for allosteric control.
- A key aspect of their design involved creating proteins with a hinge that could adopt two distinct conformations.
- This hinge acted as a flexible joint, enabling the protein to transition between different shapes in response to the presence of an effector.
- The hinge also functioned as a sensor, detecting the effector and initiating the structural change.
- Additionally, the team developed a novel method for connecting these hinge-like proteins to form larger, functional assemblies.
- By combining these techniques, they were able to create proteins with dual ridged arms and a central hinge, resulting in a stable structure that could bend in response to an effector.
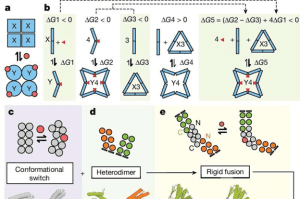 Design strategy for building switchable oligomers.
Design strategy for building switchable oligomers.
Practical Applications and Testing
- To validate their approach, the researchers created several proteins with varying shapes, each designed to respond to different effectors.
- One notable example was a protein that assembled into a ring-like structure upon interacting with a peptide-based effector.
- This experiment demonstrated the potential for designing proteins that can form complex structures in response to specific molecular triggers.
- Moreover, the team successfully engineered proteins that could bind to other proteins they had constructed, leading to the formation of entirely new structures.
- This ability to build complex protein assemblies from simpler components opens up a range of possibilities for creating molecular machines and other advanced biotechnological applications.
Implications and Future Directions
- The successful engineering of proteins with allosteric control marks a significant step forward in the field of synthetic biology.
- This technology could pave the way for the development of new therapeutic strategies, such as targeted drug delivery systems, where proteins could be designed to release their cargo only in the presence of specific cellular signals.
- Furthermore, the ability to control protein assembly and disassembly could lead to innovations in molecular machinery, where proteins act as the building blocks for nanoscale devices.
- These devices could have applications in medicine, environmental monitoring, and materials science.
- As the researchers continue to refine their techniques and explore new applications, the potential for this technology to impact various fields of science and industry remains vast.
- The work conducted by the University of Washington team is not just a technical achievement but also a glimpse into the future of protein engineering, where the precise control of molecular structures could unlock new frontiers in biology and medicine.
Allosteric regulation
- Allosteric regulation is a crucial mechanism in biochemistry where the activity of an enzyme or protein is modulated through the binding of an effector molecule at a site other than the active site.
- This binding induces conformational changes that can either enhance or inhibit the protein’s function.
- Mechanism of Allosteric Regulation
- Allosteric regulation involves two main types of effectors:
- Allosteric Activators: These molecules increase the enzyme’s activity by promoting a conformational change that enhances substrate binding.
- Allosteric Inhibitors: These decrease the enzyme’s activity by inducing a conformational change that reduces substrate binding or activity.
- The site where these effectors bind is referred to as the allosteric site, distinct from the active site where substrate binding occurs.
- This distinction allows for a more complex regulation of enzymatic activity compared to simple competitive inhibition, where inhibitors compete directly with substrates for the active site.
- Types of Allosteric Regulation
- Allosteric regulation can be categorized based on the nature of the substrates and effectors involved:
- Homotropic Regulation: This occurs when the substrate itself acts as an effector, as seen in the cooperative binding of oxygen to hemoglobin. The binding of one oxygen molecule increases the affinity of the remaining sites for oxygen.
- Heterotropic Regulation: This involves different molecules acting as effectors. For example, the binding of carbon dioxide to hemoglobin affects its oxygen-binding affinity, demonstrating how different ligands can influence the same protein’s activity
SOURCE: https://phys.org/news/2024-08-biochemists-proteins-transition-disassembly-allosteric.html
Spread the Word



