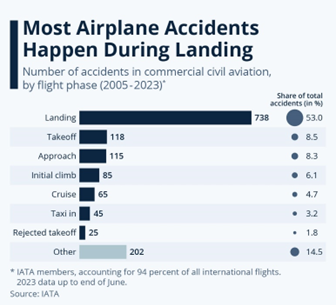
1. NYAYA VIKAS PORTAL
TAG: GS 2: JUDICIARY
THE CONTEXT: Ministry of Law and Justice bringing some new significant changes along with improvising many schemes for the Judicial services. It has also facilitated justice to common man through a plethora of initiatives like nyaya vikas portal.
EXPLANATION:
Nyaya Vikas Portal:
- This portal has been created for monitoring the implementation of this Nyaya Vikas Scheme.
- This portal provides stakeholders with convenient access to information regarding funding, documentation, project monitoring, and approval.
- Nyaya Vikas Portal created for monitoring the implementation of Centrally Sponsored Schemes for Development of Infrastructure for Judiciary.
- Nyaya Vikas Portal allows four efficient ways of logging into the portal, thereby empowering stakeholders with seamless access to information pertaining to funding, documentation, project monitoring and approval.
About Nyaya Vikas Scheme:
- The Department of Justice has been implementing the Centrally Sponsored Scheme (CSS) for Development of Infrastructure Facilities for Districts and Subordinate Judiciary since 1993-94.
- Under the Scheme, central assistance is provided to the State Government / UT Administrations for construction of court halls and residential units for Judicial Officers / Judges of District and Subordinate Courts.
- With further extension of the scheme beyond 31.03.2021, some new features like Lawyers Hall, Toilet complexes and Digital computer rooms have been added to the scheme for the convenience of lawyers and litigants, besides court halls and residential units.
- The funds sharing pattern under the Scheme for Center and State is 60:40 in respect of States other than North Eastern and Himalayan States.
- The funds sharing pattern is 90:10 in respect of North Eastern and Himalayan States; and 100% in respect of Union Territories.
- The Nyaya Vikas Program adopts a multi-faceted approach to achieve its objectives. Some of the strategies employed include targeted welfare schemes tailored to the needs of marginalized communities, capacity building through training and support, leveraging technology and digital connectivity to bridge the digital divide, and forging partnerships and collaborations with non-governmental organizations, private sector entities, and civil society.
2. DRAFT PANDEMIC TREATY AND ANTIMICROBIAL RESISTANCE
TAG: GS 2: HEALTH ISSUES
THE CONTEXT: Since the beginning of negotiations on the Pandemic Instrument, there have been calls from civil society and leading experts to include antimicrobial resistance. In the latest version of the draft Pandemic Instrument, also referred to as the “pandemic treaty,” was shared with Member States at the World Health Assembly. The text was made available online via Health Policy Watch and it quickly became apparent that all mentions of addressing antimicrobial resistance in the Pandemic Instrument were at risk of removal.
EXPLANATION:
Zero Draft of the Pandemic Treaty:
- Work on the Pandemic Instrument began in December 2021 after the World Health Assembly agreed to a global process to draft and negotiate an international instrument under the Constitution of the World Health Organization (WHO) to protect nations and communities from future pandemic emergencies.
- Zero Draft of the treaty, known as the Zero Draft of WHO CA+, was published on 1 February 2023, and discussed at the Intergovernmental Negotiating Body’s fourth meeting between 27 February 2023 and 3 March 2023.
- Because the Zero Draft is the starting point for negotiations, the substantive provisions and content of the treaty could change. But the general structure and broad issues the treaty is likely to address are more likely to remain.
- The main goal of this treaty would be to foster an all of government and all of society approach, strengthening national, regional and global capacities and resilience to future pandemics. This includes greatly enhancing international co-operation to improve, for example, alert systems, data-sharing, research and local, regional and global production and distribution of medical and public health counter-measures such as vaccines, medicines, diagnostics and personal protective equipment.
- Currently, the parties are negotiating on issues such as:
- The definition, means, and procedure for declaring a pandemic, and what this actually means in practice for states.
- How the treaty would work alongside the International Health Regulations.
- Key international principles that will guide the treaty, such as human rights, sovereignty, equity, solidarity, transparency, accountability and more.
- How to achieve equity in the global supply chain for pandemic-related products, and access to relevant technologies.
- Strengthening the resilience and responsiveness of health systems.
- How states and the WHO should be coordinating and cooperating in pandemic preparedness and response.
- How to finance pandemic preparedness and response initiatives.
- Setting up a new Governing Body for the treaty – a COP or Conference of the Parties.
- Other general legal issues relating to the treaty, such as amendments, withdrawal, and dispute settlement.
Anti Microbial Resistance:
- Since the beginning of negotiations on the Pandemic Instrument, there have been calls to include the so-called “silent” pandemic of antimicrobial resistance in the instrument as not all pandemics in the past have been caused by viruses and not all pandemics in the future will be caused by viruses. Devastating past pandemics of bacterial diseases have included plague and cholera. The next pandemic could be caused by bacteria or other microbes.
- Antimicrobial resistance (AMR) is the process by which infections caused by microbes become resistant to the medicines developed to treat them. Microbes include bacteria, fungi, viruses and parasites. Bacterial infections alone cause one in eight deaths globally.
- AMR is fueling the rise of drug-resistant infections, including drug-resistant tuberculosis, drug-resistant pneumonia and drug-resistant Staph infections such as methicillin-resistant Staphylococcus aureus (MRSA).
- Even if the world faces another viral pandemic, secondary bacterial infections will be a serious issue. During the COVID-19 pandemic for instance, large percentages of those hospitalized with COVID-19 required treatment for secondary bacterial infections.
- The exclusion of these measures would hinder efforts to protect people from future pandemics, and appears to be part of a broader shift to water-down the language in the Pandemic Instrument, making it easier for countries to opt-out of taking recommended actions to prevent future pandemics.
3. FOURTEEN FIXED-DOSE COMBINATION (FDC) MEDICINES
TAG: GS 2: HEALTH ISSUES
THE CONTEXT: Fourteen fixed-dose combination (FDC) medicines found to lack therapeutic relevance have been banned by the Central Government through a gazette notification. While industry experts claim that some of these combinations aren’t available in the market currently, the banned combinations include medicines used for cough, fever and infections, and are sold over the counter.
EXPLANATION:
- These banned drugs included those used for treating common infections, cough and fever combinations such as: Nimesulide Paracetamol dispersible tablets, Chlopheniramine Maleate Codeine Syrup, Pholcodine Promethazine, Amoxicillin Bromhexine and Bromhexine Dextromethorphan Ammonium Chloride Menthol, Paracetamol Bromhexine Phenylephrine Chlorpheniramine Guaiphenesin and Salbutamol Bromhexine.
- The expert committee said that there is “no therapeutic justification for this FDC (fixed dose combination) and the FDC may involve risk to human beings.
- Hence, in the larger public interest, it is necessary to prohibit the manufacture, sale or distribution of this FDC under section 26 A of the Drugs and Cosmetics Act, 1940.
Fixed-Dose Combination (FDC) medicines:
- According to the Central Drugs Standard Control Organisation (CDSCO), FDCs refer to products containing one or more active ingredients used for a particular indication(s).
- FDCs can be divided into the following groups and data required for approval for marketing is described below:
- The first group of FDCs includes those in which one or more of the active ingredients is a new drug. For such FDCs to be approved for marketing data to be submitted will be similar to data required for any new drug (including clinical trials).
- The second group FDCs includes those in which active ingredients already approved/marketed individually are combined for the first time, for a particular claim and where the ingredients are likely to have significant interaction of a pharmacodynamic or pharmacokinetic nature.
- The third group of FDCs includes those which are already marketed, but in which it is proposed either to change the ratio of active ingredients or to make a new therapeutic claim. For such FDCs, the appropriate rationale including published reports should be submitted to obtain marketing permission. Permission will be granted depending upon the nature of the claim and data submitted.
- The fourth group of FDC includes those whose individual active ingredients (or drugs from the same class) have been widely used in a particular indication(s) for years, their concomitant use is often necessary and no claim is proposed to be made other than convenience. It will have to be demonstrated that the proposed dosage form is stable and the ingredients are unlikely to have significant interaction of a pharmacodynamic or pharmacokinetic nature. No additional animal or human data are generally required for these FDCs, and marketing permission may be granted if the FDC has an acceptable rationale.
Central Drugs Standard Control Organisation (CDSCO):
- It is under Directorate General of Health Services,Ministry of Health & Family Welfare,Government of India.
- Its headquarter is located at FDA Bhawan, Kotla Road, New Delhi and also has six zonal offices,four sub zonal offices,thirteen Port offices and seven laboratories spread across the country.
- The Drugs & Cosmetics Act,1940 and rules 1945 have entrusted various responsibilities to central & state regulators for regulation of drugs & cosmetics.
- It envisages uniform implementation of the provisions of the Act & Rules made there under for ensuring the safety, rights and well being of the patients by regulating the drugs and cosmetics.
- Under the Drugs and Cosmetics Act, CDSCO is responsible for approval of Drugs, Conduct of Clinical Trials, laying down the standards for Drugs, control over the quality of imported Drugs in the country and coordination of the activities of State Drug Control Organizations by providing expert advice with a view of bring about the uniformity in the enforcement of the Drugs and Cosmetics Act.
- Further CDSCO along with state regulators, is jointly responsible for grant of licenses of certain specialized categories of critical Drugs such as blood and blood products, I. V. Fluids, Vaccine and Sera.
- The Central Drugs Standard Control Organization (CDSCO) is the primary regulatory body for medical devices in India. It is responsible for overseeing the import, manufacture, sale, and distribution of medical devices in the country.
4. THE DECADE-LONG SEARCH FOR A RARE HIGGS BOSON DECAY
TAG: GS 3: SCIENCE AND TECHNOLOGY
THE CONTEXT: Recently, physicists working with the Large Hadron Collider (LHC) particle-smasher at CERN, in Europe, reported that they had detected a Higgs boson decaying into a Z boson particle and a photon. This is a very rare decay process that tells us important things about the Higgs boson as well as about our universe.
EXPLANATION:
- The Higgs boson is a type of boson, a force-carrying subatomic particle.
- It carries the force that a particle experiences when it moves through an energy field, called the Higgs field, that is believed to be present throughout the universe.
- For example, when an electron interacts with the Higgs field, the effects it experiences are said to be due to its interaction with Higgs bosons
- The stronger a particle’s interaction with the Higgs boson, the more mass it has. This is why electrons have a certain mass, protons have more of it, and neutrons have just a little bit more than protons, and so on.
- A Higgs boson can also interact with another Higgs boson: this is how we know that its mass is greater than that of protons or neutrons.
- Photons, the particles of light, have no mass because they don’t interact with Higgs bosons
How did a Higgs boson decay to a Z boson and a photon if it doesn’t interact with photons and what are virtual particles?
- According to quantum field theory, which is the theory physicists use to study these interactions, space at the subatomic level is not empty. It is filled with virtual particles, which are particles that quickly pop in and out of existence. They can’t be detected directly, but according to physicists their effects sometimes linger.
- The LHC creates a Higgs boson by accelerating billions of highly energetic protons into a head-on collision, releasing a tremendous amount of energy that condenses into different particles.
- When a Higgs boson is created in this hot soup, it has a fleeting interaction with virtual particles that creates a Z boson and a photon.
What is the new result?
- Higgs boson is an unstable particle because it is so heavy, that decays into lighter particles.
- For example, this theory, called the Standard Model, says that a Higgs boson will decay to a Z boson and a photon 0.1% of the time. This means the LHC needed to have created at least 1,000 Higgs bosons to have been able to spot one of them decaying to a Z boson and a photon.
- As it happens, the Z boson is also unstable. Z bosons decay to two muons some 3% of the time. If the detectors at the LHC were looking for a pair of muons plus a photon created at the same time, the LHC would have had to create at least 30,000 Higgs bosons to observe the decay just once.
- The two detectors that announced the new measurement, called ATLAS and CMS, had in fact looked for and found the decay before as well (in 2018 and 2020). On this occasion, however, the two teams combined their data, collected “between 2015 and 2018”, and as a result “significantly increased the statistical precision and reach of their searches,” according to a CERN statement.

What is the Standard Model?
- The Standard Model has made many accurate predictions but it can’t explain what dark matter is or, in fact, why the Higgs boson is so heavy. Testing its predictions as precisely as possible is a way for physicists to find whether there are any cracks in the Model – cracks through which they can validate new theories of physics.
- Standard Model predicts that the Higgs boson will take this path 0.1% of the time if its mass is 125 billion eV/c2 (a unit of mass used for subatomic particles).
5. THE ENERGY PROGRESS REPORT 2023
TAG: PRELIMS PERSPECTIVE
THE CONTEXT: According to the energy progress report 2023, World still off-track from achieving universal energy access to all i.e SDG 7. High inflation, debt distress, policy inactions and lagging financial flows slowing access to electricity, clean cooking in developing economies
EXPLANATION:
Finding of the report:
- SDG 7 includes reaching universal access to electricity and clean cooking, doubling historic levels of efficiency improvements, and substantially increasing the share of renewables in the global energy mix.
- Several major economic factors are impeding the realisation of SDG 7 globally, like uncertain macroeconomic outlook, high levels of inflation, currency fluctuations, debt distress in many countries, lack of financing, supply chain bottlenecks, tighter fiscal circumstances and soaring prices for materials.
- The rate of improvement in energy efficiency (target 7.3) is not on track to double by 2030, with the current trend of 1.8 per cent falling short of the targeted increase of 2.6 per cent each year between 2010-2030.
- Globally, access to electricity grew by an annual average of 0.7 percentage points between 2010 and 2021, rising from 84 per cent of the world’s population to 91 per cent.
- With the ongoing impact of COVID-19 and soaring energy prices, the IEA estimates show 100 million people who recently transitioned to clean cooking may revert to using traditional biomass, the report added.
- In 2020, the share of renewable energy in total final energy consumption stood at just 19.1 per cent (or 12.5 per cent if traditional use of biomass is excluded), not much more than the 16 per cent a decade earlier.
Sustainable Development Goal:
- Sustainable Development Goals are the blueprint to achieve a better and more sustainable future for all.
- They address the global challenges we face, including those related to poverty, inequality, climate change, environmental degradation, peace and justice.
- The 17 Goals are all interconnected, and in order to leave no one behind, it is important that we achieve them all by 2030.

Energy Progress Report:
- IRENA produces the report jointly with the SDG 7 co-custodian agencies: the International Energy Agency (IEA), the United Nations Statistics Division (UNSD), the World Bank, and the World Health Organization (WHO).
- The Energy Progress Report provides the international community with a global dashboard to register progress on the targets of Sustainable Development Goal 7 (SDG7): ensuring universal energy access, doubling progress on energy efficiency and substantially increasing the share of renewable energy.
- It also registers progress towards enhanced international cooperation to facilitate access to clean and renewable energy by 2030, as well as on the expansion of infrastructure and technology upgrade for supplying modern and sustainable energy services for all in developing countries.
- It assesses the progress made by each country on these targets and provides a snapshot of how far we are from achieving SDG7. The 2022 release is the eight edition of this report, which was formerly known as the Global Tracking Framework (GTF).
International Energy Agency:
- It is an international energy forum comprised of 29 industrialized countries under the Organization for Economic Development and Cooperation (OECD).
- The IEA was established in 1974, in the wake of the 1973-1974 oil crisis, to help its members respond to major oil supply disruptions, a role it continues to fulfill today.
- IEA’s mandate has expanded over time to include tracking and analyzing global key energy trends, promoting sound energy policy, and fostering multinational energy technology cooperation.
International Renewable Energy Agency (IRENA):
- It is an intergovernmental organisation that supports countries in their transition to a sustainable energy future, and serves as the principal platform for international cooperation, a centre of excellence, and a repository of policy, technology, resource and financial knowledge on renewable energy.
- IRENA promotes the widespread adoption and sustainable use of all forms of renewable energy, including bioenergy, geothermal, hydropower, ocean, solar and wind energy in the pursuit of sustainable development, energy access, energy security and low-carbon economic growth and prosperity.